A Covalent Bond Is Best Described as
C a bond between a metal and a nonmetal. The electron pairs that are.
The atoms valence electrons are shared between the atoms.

. The transfer of electrons. Atoms from different elements will not share electrons because their outer shells are full. A covalent bond can be best described as a bond between to atoms which share electrons.
A covalent bond is best described as Select one. 421 Describe the covalent bond as the electrostatic attraction between a pair of electrons and positively charged nuclei. Up to 24 cash back Test.
Identify the choice that best completes the statement or answers the question. The force that holds atoms together is referred to as a _____. D the transfer of electrons.
The C-Cl bond is best described as A. This is different from ionic bonds where electrons are taken from one atom and placed. This is different from ionic bonds where electrons are taken from one atom and placed.
A the sharing of electrons between atoms. A covalent bond is described as the relationship created when electrons are shared between atoms. 5 Give the name for SnO.
Two atoms sharing electrons. Neutral atoms coming together to share electrons. A covalent bond is formed by the equal sharing of electrons from both the participating atoms.
D a bond between a metal and a polyatomic ion. C the sharing of electrons between atoms. с A chemical bond in which four or more electrons are shared.
Atoms get covalently bond with different atoms to obtain more stability that is obtained by creating a complete electron shell. Valence electrons are transferred from one atom to the other. Covalent bonds can be best described as.
Which statement correctly describes a covalent bond. E a bond between two polyatomic ions. All of the atoms electrons are shared between the atoms.
A bond between two polyatomic ions. A bond between a metal and a nonmetal. The diagrams below show the.
Phosphorous P and chlorine Cl bond covalently to form the important industrial compound phosphorous trichloride. Elements with a high electronega. E a bond between a metal and a polyatomic ion.
A bond between two polyatomic ions. A covalent bond is best described as. 2 A covalent bond is best described as.
A chemical bond in which one atom loses an electron Which statement best describes a covalent bond. B A chemical bond that involves sharing a pair of electrons between atoms in a molecule. One atom losing a proton to another then sticking to it due to the attraction between opposite charges.
Covalent bonding is formed when the pairs of electrons are shared via atoms and they are called a molecular bond. Which phrase best describes a covalent bond between two atoms. A covalent bond occurs when atoms _____ electrons.
A covalent bond can best be described as A. A A chemical bond in which no electrons are shared. A bond between two polyatomic ions.
The sharing of electrons between atoms. The pair of electrons participating in this type of bonding is called shared pair or bonding pair. Covalent bonds can be best described as neutral atoms coming together to share electrons.
B the transfer of electrons. B a bond between two polyatomic ions. 31 a covalent bond can best be described as a two.
A bond between two polyatomic ions. The sharing of electrons between atoms. The atoms valence electrons combine to form a network of bonds.
The sharing of electrons between atoms. This is often created by two nonmetals. The sharing of electrons between atoms.
The polar covalent bond is a type of covalent bond formed between two non-identical atoms. Two atoms sharing protons. The transfer of electrons.
A bond between a metal and a nonmetal. 16 16 Molecules can be described as A Two or more pure substances fully mixed. One atom losing an electron to another then sticking to it due to the attraction between opposite charges.
A bond between a metal and a polyatomic ion. A covalent bond can be best described as a bond between to atoms which share electrons. 15 15 A covalent bond is best described as A a bond between a metal and a nonmetal.
A bond between a metal and a polyatomic ion. Up to 256 cash back A covalent bond is best described as. The transfer of electrons.
Covalent Bonding Multiple Choice Directions. The transfer of electrons. A bound between a metal and a polyatomic ion.
Identify the compound with covalent bonds. A bond between a metal and a polyatomic ion. A bond between a metal and a nonmetal.
The right answer is electron sharing. A covalent bond is best described as a bond between a metal and a nonmetal.

Drill Ionic Bonding Objective Ppt Download

Solved An Ionic Bond Is Best Described As A The Attraction Chegg Com

Covalent Bonds Flashcards Quizlet
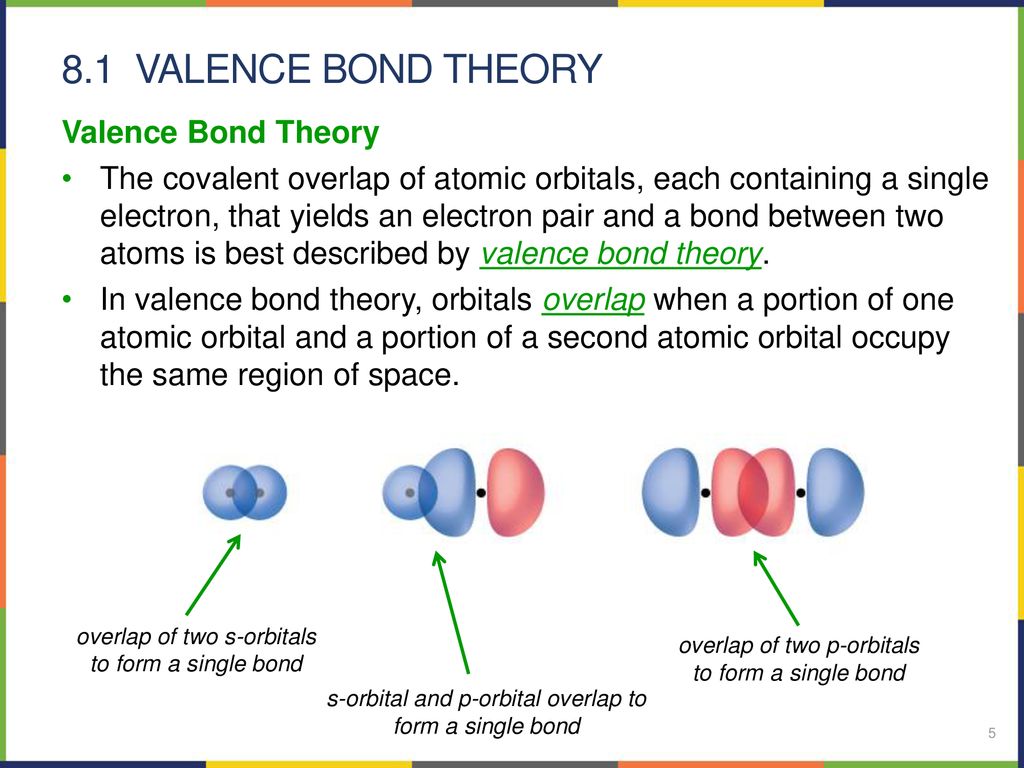
Chemistry Advanced Theories Of Covalent Bonding Chapter 8 Ppt Download

1 Structure And Bonding Why This Chapter Ppt Download

Pin On Full Download Test Bank For Fundamentals Of General Organic And Biological Chemistry 7th Edition By

Solved 6 A Chemical Bond Formed When Two Atoms Share Two Chegg Com

When A Chemical Bond Is Broken Energy Is Ppt Download

Bond Polarity Flashcards Quizlet

Che 140 Ch 8 Learn Smart Flashcards Quizlet

Covalent Bonds Flashcards Quizlet

Do Now Which Substance Contains Bonds That Involved The Transfer Of Electrons From One Atom To Another Co2 Nh3 Kbr Cl2 When Sodium And Fluorine Combine Ppt Video Online Download

Ib Chemistry Year 1 Hl The Brooklyn Latin School Ppt Download

Nuclear Chain Reaction Physical Chemistry Chemistry Physics

Question 6 Of 10 Look At The Picture Below Which Best Describes The Bond Shown A A Covalent Bond Brainly Com